With a constant demand to meet global regulations, maintain corporate responsibility in manufacturing and keep your products on the market and available to your customers, it may feel like the effort to gather product data is endless. Constantly collecting data and resurveying your supply chain when new substances are added to regulations or customer requests is not an efficient way to do business — leading to lost time, redundancies and frustration.
DXC Technology, the company behind the automotive industry’s International Material Data System (IMDS), has developed the Compliance Data Exchange (CDX) to meet the need for a robust product compliance data collection and reporting system across industries. After 20 years of seeing the value of IMDS in the automotive industry, they recognized that other industries could use the power of a centralized database and full materials declarations to more easily meet industry regulations and customer requests.
Whether or not you are already using IMDS, you may be wondering why your customers are requesting data entry into CDX or how to get started using CDX. We’ve compiled this list of the 10 most frequently asked questions about CDX to help you learn more and understand how CDX can fit into your compliance reporting.
CDX Frequently Asked Questions
1. I supply the same product to multiple industries and my data is already in IMDS. My non-automotive customer is requesting a CDX submission. Can’t they just register in IMDS and get my data from there?
No. Per the IMDS Terms of Use, only authorized automotive OEMs and their direct supply chain can use IMDS. If your customer is non-automotive and not part of the automotive community, they cannot use IMDS. You will need to re-enter the data into CDX or purchase the necessary licenses to transfer data from IMDS to CDX using the data transfer option.
2. My customer is asking for a CDX submission, how do I get started?
The best way to get started is through education and training. There are multiple resources directly on the CDX website or you could consider taking Tetra Tech’s hands-on, online CDX Training.
3. What regulations or substance of concern lists does CDX help me comply with?
CDX can support you in meeting a variety of regulations:
- REACH Candidate List
- RoHS
- ELV
- GADSL
- Batteries
- Packaging
- International Maritime Organization (IMO)
- REACH Annex XIV and XVII
- Aerospace and Defense Declarable Substance List (AD-DSL)
- California Proposition 65
In addition, CDX can also support conflict minerals reporting, SCIP database reporting and customer-specific requirements.
4. Does CDX have a way to send data requests to suppliers?
Yes. A user can use the MDS Request feature to request data from suppliers. Requests can be made to both registered and unregistered suppliers. Unregistered suppliers will receive an e-mail with registration details. Bulk requests can be made to registered suppliers.
5. My customer sent me a request for CDX and requires FMD, what does that mean?
FMD stands for full material declaration or 100% declaration. FMD will provide your customer with the necessary details to determine compliance. It is likely your customer will not have to request additional information from you when regulations, exemptions or substances of concerns change year after year. FMD requires the disclosure of each homogenous material used in your product and each material’s substance composition. If you have concerns regarding proprietary formulations, CDX has options for marking substances as confidential.
6. How do I know if my product is compliant?
Unfortunately, CDX cannot tell you whether or not a product is compliant; it will still require due diligence and compliance expertise. However, CDX does include multiple options to help the user evaluate compliance. These options include data checks identifying warnings and errors, where-used analysis reports, and data filtering for specific regulations and substance lists.
7. If my product contains a RoHS substance but has a valid RoHS exemption, how do I apply the exemption to the data in CDX?
First, make sure that “RoHS” is in scope for your company under Administration (Click “Administration” and then “Company” to verify). Second, open the product datasheet in Edit mode and expand the Regulations section of the product details for each component containing the RoHS substance in question. Right click in the blank cell under Exemption and select “Edit.”
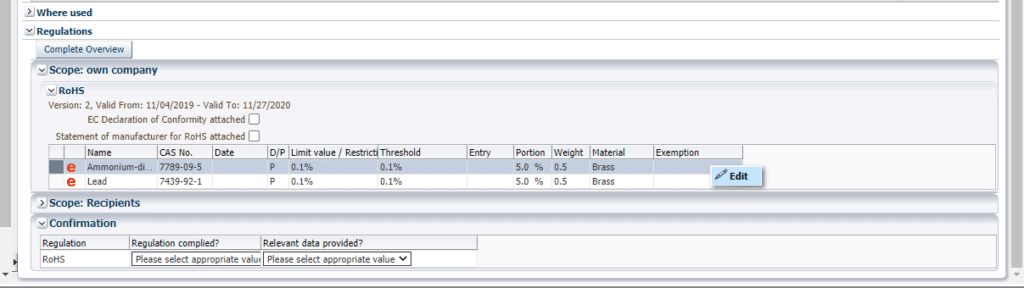
A new window will open where you can change the “unknown” exemption to a valid exemption (right click, edit). Finally, select the appropriate list and exemption, and click “Apply.”
8. Can I attach supporting documentation to my CDX entry?
Yes. You can attach up to five documents (up to 10 MB per file) in the following formats: xls, xlsx, doc, docx, pdf or zip. Refer to the “Attachment” section of the product details in the datasheet. Click “Add file” to search for and select the file(s) you would like to attach.
9. How do I build a simple part in CDX that is made from a single standard material?
Once you are logged into CDX, select the MDS pull-down and click “New” then “Component.” Complete the required fields and save. Click the “Add material” button and search for and apply the Standard material. Enter the material weight (same as the product weight) and complete other relevant fields.
10. Is CDX free?
Yes. It is free to create and submit a datasheet and to create a conflict minerals declaration. However, there are some features that are only available with a paid MDS or CMD license, such accepting and rejecting supplier MDS and creating internal substance lists and regulations.
Get CDX Support
CDX is a powerful tool that can assist you in meeting regulations across a variety of industries. If you are just getting started, Tetra Tech can help you understand how to use CDX to build full materials declarations, saving you time in the future as new regulations and requirements are added to your compliance demands.
We offer a basic CDX training for those wanting to build a solid foundation with CDX. We can also answer other CDX questions and support you in meeting regulatory requirements. Contact us today to get answers to you CDX questions, learn more about our CDX training or to see how we can support your compliance needs.







